News 2025-07-31
Porton and Repare Therapeutics Collaborate on OPRD Publication, Demonstrating Strength in Synthetic Process Development and Optimization
Recently, Repare Therapeutics and Porton Pharma Solutions Ltd. (Hereinafter referred to as “Porton” or the company “) published the paper “Scalable Synthesis of Lunresertib, a selective PKMYT1 Inhibitor” in the authoritative publication “Organic Process Research & Development” (OPRD).
In this research, the Repare and Porton teams developed an efficient, robust, and scalable route for the synthesis of Lunresertib (RP-6306), supporting rapid production of the corresponding API. The synthesis features two palladium-catalyzed couplings, a novel chiral resolution of atropisomers, a one-pot hydration/demethylation sequence, and a recrystallization that improves enantiomeric purity. This achievement highlights Porton’s expertise in process optimization and technology transfer.

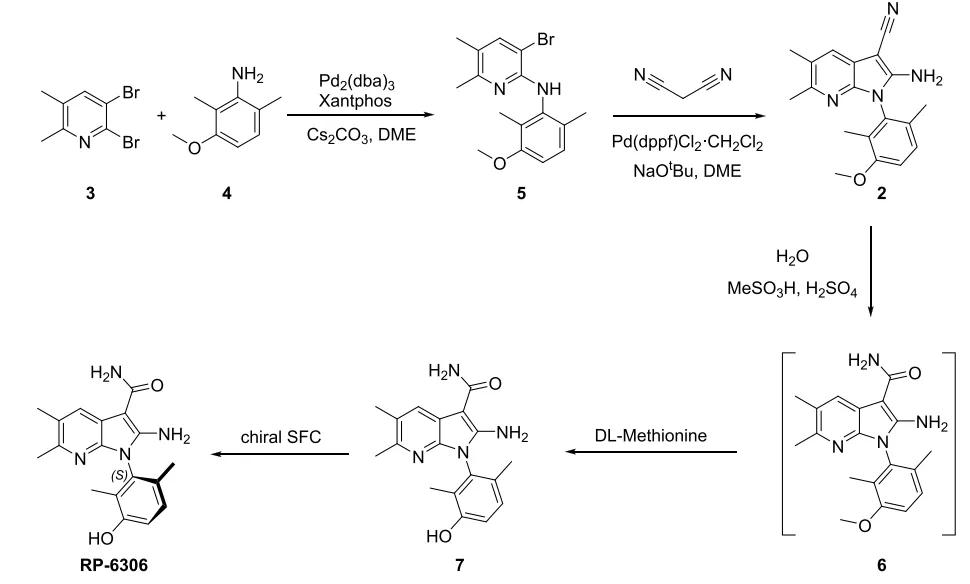
PKMYT1 is a regulator of CDK1 phosphorylation and is a compelling therapeutic target for the treatment of certain types of DNA damage response cancers due to its established synthetic lethal relationship with CCNE1 amplification. Lunresertib is a selective and orally bioavailable PKMYT1 inhibitor, which is currently bening evaluated in Phase 1 clinical trials for the treatment of genetically selected solid tumors. Lunresertib drug substance RP-6306 contains a chiral atropisomeric axis, which arises from restricted rotation around the C-N bond between the azaindole and dimethylphenol portions. Only the S-isomer is PKMYT1 active, while the corresponding R-isomer is inactive. In early stage, Porton's process R&D and the Supercritical Fluid Chromatography (SFC) technical team provided the desired S-isomer in gram scale to support the preclinical studies (Scheme 1). While this route provided a quality-assured API for early-stage research, the process was inefficient, long cycle time and high cost. Hence, the development of an efficient method to access the desired S-isomer is crucial before scaling up the preparation of RP-6306.
Scheme
1. Medicinal Chemistry Approach to RP-6306
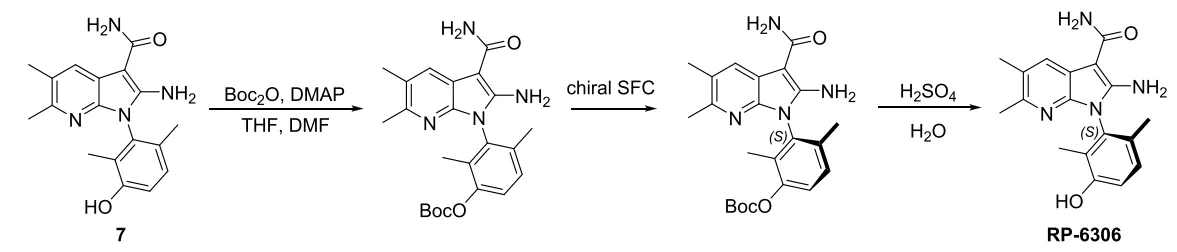
Compound 7 is known to have low solubility in the solvents commonly used for SFC chromatography, which resulted in a low efficiency for SFC separation. Therefore, in the early stage of the process development, Porton's process R&D and SFC team derived compound 7 with a Boc group to increase the solubility to allow more efficient isolation of the S-enantiomer, which was deprotected to give RP-6306. However, this method involves more synthetic steps and has limited improvement in efficiency, which fails to fundamentally solve the problems of having a long cycle time and high cost.
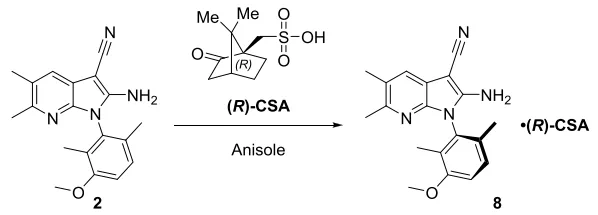
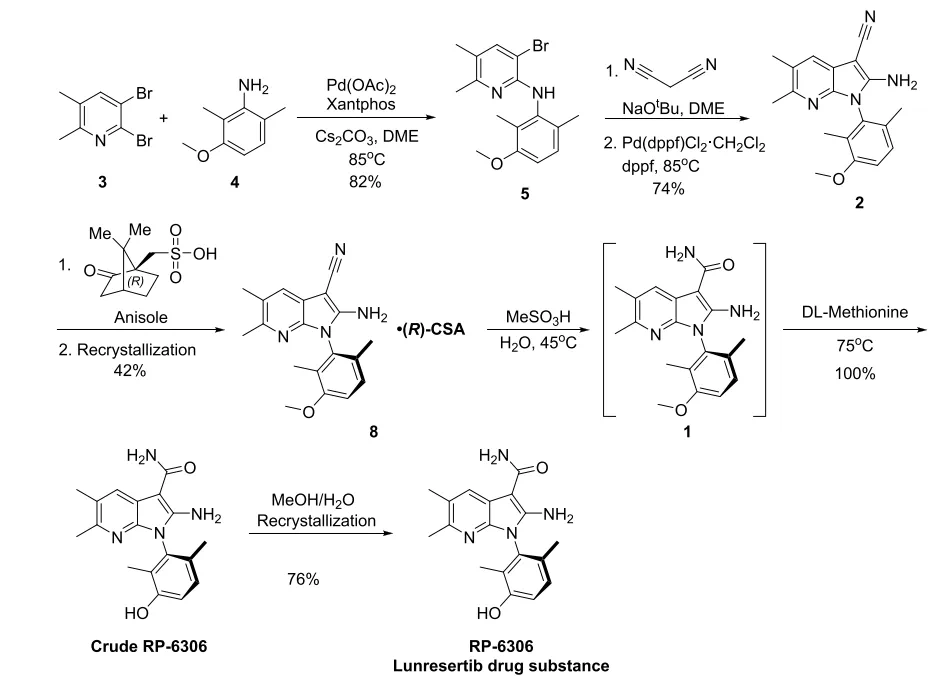
Considering intermediate 2 contains basic amino acid groups, we focus the feasibility on the classical chiral salt resolution. After screening about 150 salt/solvent combinations, the scientist at J-STAR Research Inc., a subsidiary of Porton, finally identified the most promising resolution condition with chiral acid(R)-CSA in anisole. Subsequently, Porton's process R&D team screened the crystallization temperature, solvent amount, crystallization aging time, and times of crystallizations to confirm the range of process parameters for salt-forming chiral resolution.
After establishing a scalable approach to enhance chiral purity, the Porton process R&D team explored the remaining steps to reduce the manufacturing costs.
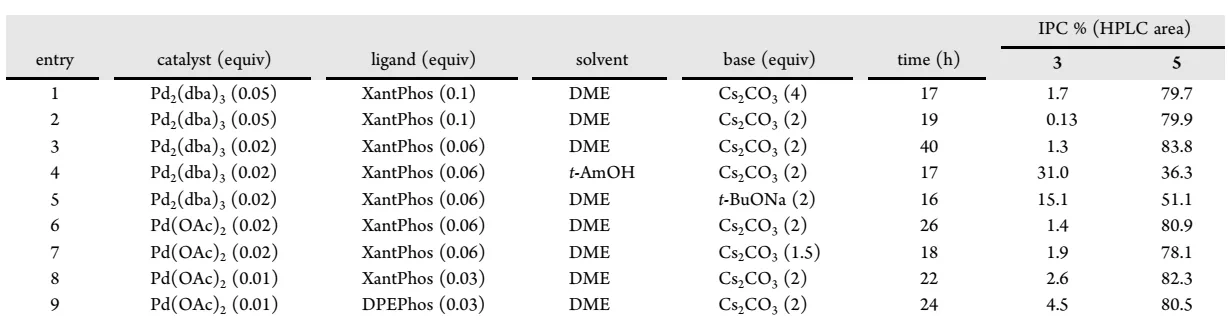
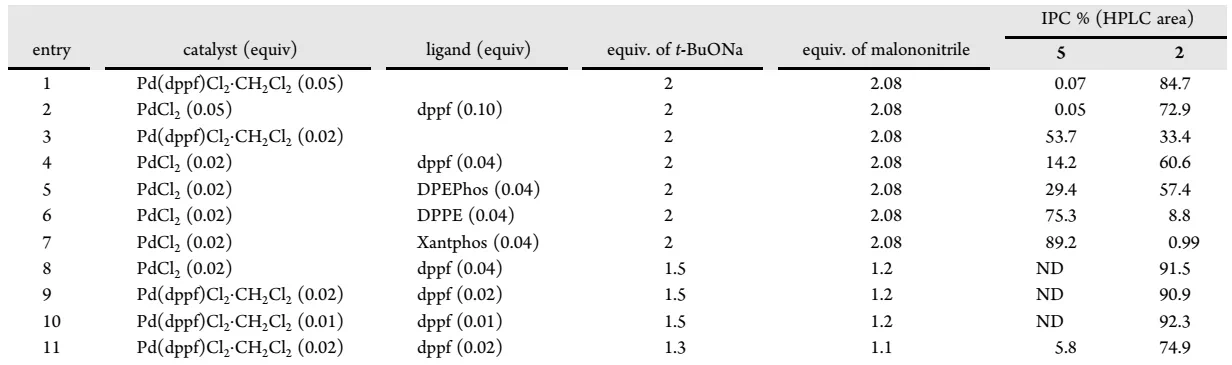
As shown in the initial synthetic route, intermediate 2 is synthesized through the Buchwald-Hartwig coupling of dibromide 3 with aniline 4 followed by cyclization with malononitrile. Both reactions worked well with >70% isolation yields. The optimization results of the first step are summarized in following table. Direct substitution has been attempted previously for the synthesis of compound 5, but it did not work as well as the palladium-catalyzed reaction. When Pd2(dba)3 is replaced with Pd(OAc)2, the catalyst loading can be reduced to 0.01 equivalent, and the amount of base can be reduced to 1.5 equivalent.
For the second step reaction, Porton’s Catalyst Screening Platform initially explored alternative catalytic reaction condition using copper instead of palladium for the aza-indole synthesis. However, copper catalyzed reaction gave much worse conversion. Therefore, the Porton process R&D team focused on the optimization of palladium-catalyzed reactions. Interestingly, direct reduction of catalyst loading will lead to an incomplete transformation; but while a lower amount of malononitrile is added, the reaction can be completed under lower usage of the catalyst. Since Pd(dppf)Cl2·CH2Cl2 is more stable than PdCl2, the condition entry 9 in the following table was finally selected for this step.
For preparation of RP-6306 from compound 8, the Porton Process R&D team initially proceeded via a two-step reaction, as the final API obtained from the one-pot procedure was only about 96% HPLC purity. In the two-step method, compound 8 was converted to compound 1 with quantitative yield by heating in methanesulfonic acid and water under 40°C for 1 hour. Compound 1 was demethylated with DL-Methionine in methanesulfonic acid to obtain RP-6306 with 92% yield and 98.8% HPLC purity. However, both reactions required large amount of methanesulfonic acid. This leads to a large amount of waste liquid generated in quenching process.
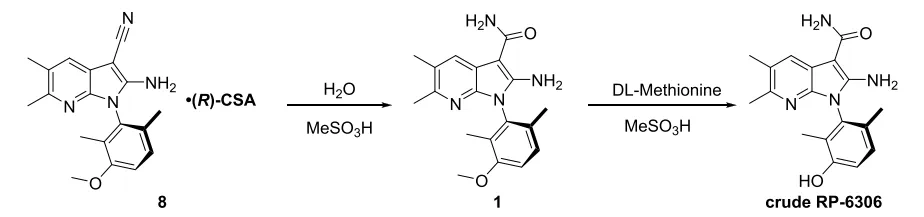
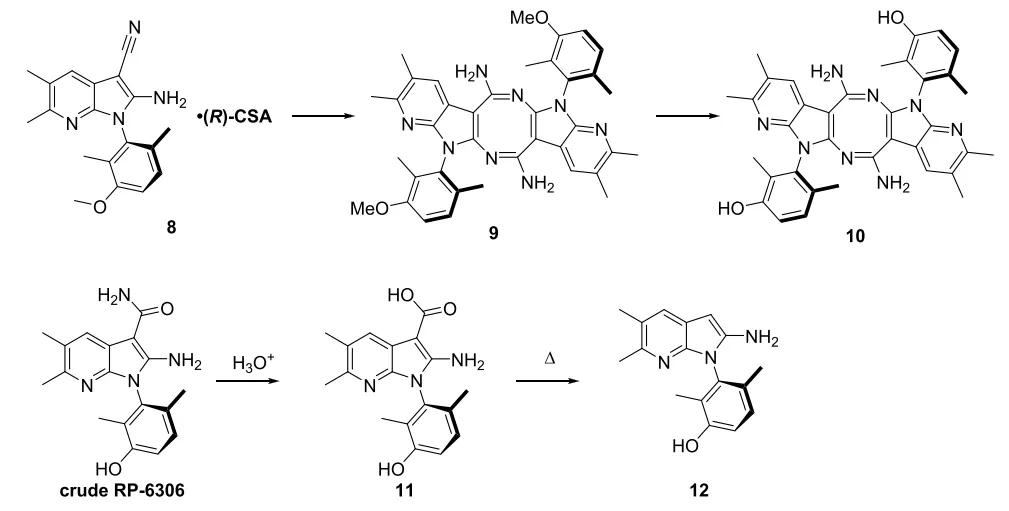
To reduce the output of waste liquid, the Porton Process R&D team re-considered the one-pot procedure to avoid the separation of compound 1. The first step was to confirm the cause of low purity in the one-pot procedure, dimer 10 and decarboxylation impurities 12. Decarboxylation likely occurred during the demethylation to give impurity 12. Dimer 10 is poorly soluble; hence it cannot be effectively removed from crude RP-6306 by recrystallization. After a series of optimizations, these two impurity levels were reduced and effectively controlled.
The final process of RP-6306 is shown in the following scheme. The Buchwald-Hartwig amination reaction followed by intramolecular cyclization reaction gave azaindole 2. Chiral resolution using (R)-CSA followed by a single round of recrystallization in anisole led to chiral 8 in 42% yield with 97.4% chiral purity on >100 kg scale. Crude RP-6306 was obtained from 8 in one pot cleanly by careful control of the amount of water and portion-wise addition of 8. Recrystallization in methanol with removal of the S/R (3:1) mixture and decolorization with activated carbon upgraded the quality of final API to >99.5% chiral purity and >99% HPLC purity on >30 kg scale.
As a globally recognized leader in the CDMO, Porton has joined forces with Repare Therapeutics to unveil their research outcomes at OPRD (Organic Process Research & Development). This milestone was achieved through Porton's extensive experience and deep-rooted expertise in small molecule drug services over the years. The R&D team's relentless pursuit of process optimization, coupled with the transformative impact of cutting-edge technologies such as metal catalysis and SFC, along with seamless collaboration across multinational teams, collectively facilitated the successful scale-up of the process at Porton's production facility.
This achievement not only highlights Porton's technological prowess spanning the entire R&D-to-production continuum but also reinforces its fundamental value in global pharmaceutical partnerships: we remain committed to supporting our clients in overcoming R&D hurdles for novel drugs through innovative and dependable CMC solutions, thereby enabling the public’s early access to good medicines.
Others
More
News 2025-09-28
Porton Shanghai Pudong Facility Obtained ISO 14001 and ISO 45001 Certification
Porton Pharma's Shanghai Pudong site successfully obtained ISO 14001 and ISO 45001 certifications, enhancing sustainable operations and CDMO capabilities for biotech clients.

News 2025-09-25
Porton Pharma Makes China's Innovative Pharmaceuticals Decennial Honor Roll, Secures 'Industry-Leading CDMO' Title
Porton Pharma honored as Industry Leading CDMO Company for innovative drug development services. Learn more about our award and global capabilities.

