News 2025-06-20
Porton Shanghai Pudong R&D and Manufacturing Site Has Passed the EU QP Audit
Porton Pharma Solutions Ltd. (hereinafter “Porton”) is glad to announce that its Shanghai Pudong R&D and Manufacturing Site has passed the EU Qualified Person (QP) audit.

This three-day audit, led by a German QP Auditor, covers quality management, production management, plant equipment and facilities, material systems, and packaging and labeling systems. The GMP Compliance Statement declares that the quality system and facility of Shanghai Pudong R&D and Manufacturing Site are in compliance with the standard of EU GMP Guidelines EudraLex Volume 4, EU Clinical Trial Regulation, GMP for IMP and ICH Q7/Q9/Q10, laying a strong foundation for Porton to further expand its global new molecular business.
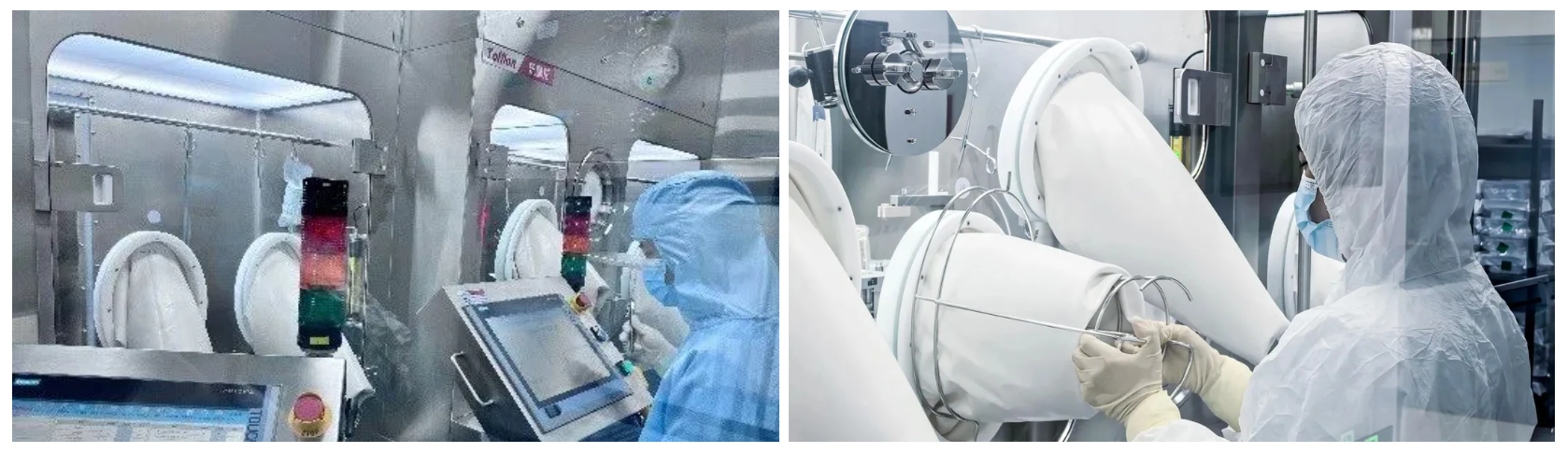
Shanghai Pudong R&D and Manufacturing Site operates R&D and manufacturing facilities, offering one-stop Biologics and Conjugates CDMO services from Drug Substance to Drug Product. Porton has established the ability to provide CDMO solutions for biologics and conjugates, including ADCs, AOCs, PDCs, RDCs and more for our customers.
Over the past 20 years, Porton has been deeply involved in the global pharmaceutical CDMO, especially accumulating rich experience in the European market. With long-term practice in providing services to numerous European clients, Porton is fully familiar with European market regulations, has good project delivery records, and continuously optimizes its service system to more accurately grasp the needs of the European market. After Shanghai Fengxian Manufacturing Site, Shanghai Pudong R&D and Manufacturing Site has also successfully passed the QP audit, which is a continuous verification of Porton's quality system's compliance with international regulatory requirements. It has once again proved that Porton has established an effective and reliable quality management system and has a professional and excellent quality management team.
In the future, Porton will continue to adhere to the quality management principle of "One Porton, One Quality", and integrate quality management throughout the entire lifecycle of drugs, providing high-level end-to-end CDMO services for global customers, and continuing to make efforts in the European new molecule business market and explore greater potential.
Others
More
News 2025-06-19
Porton Shanghai Fengxian Manufacturing Site Has Passed the EU QP Audit
The successful completion of the QP audit is a recognition of the quality management system of Shanghai Fengxian Manufacturing Site, demonstrating Porton's commitment to practicing high standard quality management. It marks that Shanghai Fengxian Manufacturing Site can provide CDMO services for small molecules, peptides and oligonucleotides, proteins and conjugate drugs that meet international standards to global customers.

News 2025-06-13
Porton Awarded Gold Medal by EcoVadis, Ranked Among the Top 5% Globally
As a key participant in the global pharmaceutical value chain, Porton is dedicated to embedding sustainability into its corporate strategy and operations.

